Breaking News

Popular News

Discover the fundamentals of chemistry, from atoms to the periodic table and chemical reactions. Explore the importance of chemistry in everyday life.Are you curious about the fundamental building blocks of matter? Chemistry is the science that delves into the structure, composition, and properties of substances, and it plays a crucial role in understanding the world around us. In this blog post, we will explore the basics of chemistry and provide an introduction to the fascinating world of atoms, chemical reactions, elements, compounds, the periodic table, and molecular structures. By understanding these concepts, we can gain insight into the importance of chemistry in our everyday lives. Whether you’re a student, a science enthusiast, or simply someone looking to grasp the fundamentals of this essential science, this blog post will serve as a helpful guide to unraveling the mysteries of chemistry. Let’s dive in and uncover the secrets of the microscopic world that shapes the macroscopic world we live in.
Contents
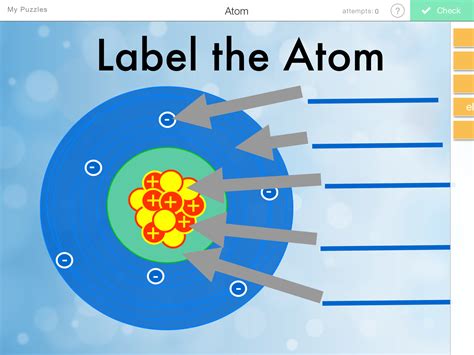
Atoms are the fundamental building blocks of matter. They are the smallest particles of an element that retain the chemical properties of the element. Everything around us, from the air we breathe to the food we eat, is made up of atoms. These tiny particles make up the world as we know it, and understanding their structure and behavior is crucial to understanding the world of chemistry.
Each atom is made up of three basic components: protons, neutrons, and electrons. The protons and neutrons are located in the nucleus of the atom, while the electrons orbit around the nucleus in energy levels. The number of protons in the nucleus determines the element’s identity, and the overall number of protons and neutrons in the nucleus determines the atom’s mass. The arrangement of electrons in the energy levels determines the chemical behavior of the atom.
Atoms can combine with other atoms to form molecules through chemical reactions. These reactions involve the gaining and losing of electrons or the sharing of electrons between atoms. The way in which atoms interact with each other and combine to form molecules is the basis for all the chemical processes that occur in the world around us. Understanding the basics of atoms is crucial to understanding the foundational principles of chemistry.
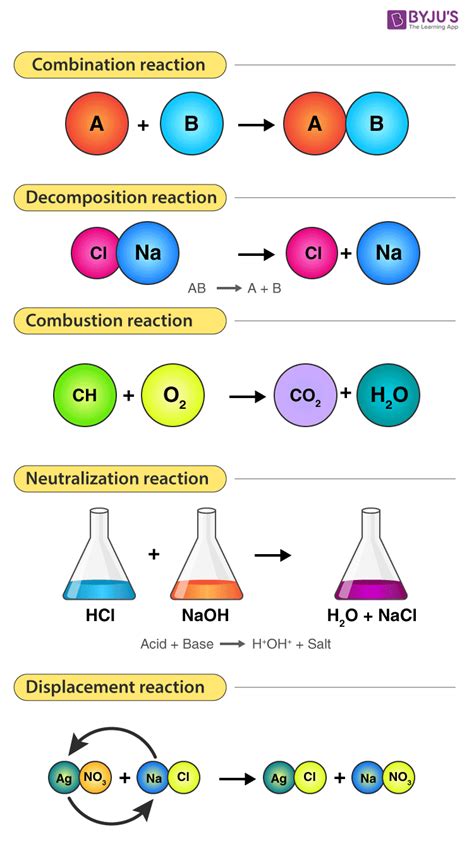
Chemical reactions are the processes that result in the formation of new substances or the breaking down of existing ones. These reactions involve the rearrangement of atoms to form new molecules with different properties. Understanding how chemical reactions work is crucial in fields such as medicine, agriculture, and environmental science, as well as in everyday life.
Chemical reactions occur when atoms combine with one another, either by sharing electrons or by transferring them. These interactions can lead to the formation of ionic or covalent bonds, resulting in the creation of compounds with unique characteristics. The study of chemical reactions helps scientists to predict the outcome of specific reactions and develop new materials with desired properties.
One way to understand chemical reactions is by examining the changes in energy that occur during the process. Some reactions release energy, while others absorb it. The rate at which a reaction takes place can also provide valuable insights into the underlying mechanisms. By studying these factors, chemists can gain a deeper understanding of how different substances interact with one another.
Chemical reactions play a vital role in numerous natural and industrial processes. From the digestion of food in our bodies to the production of materials in factories, the ability to understand and control chemical reactions is essential for progress and innovation in various fields. By learning about the fundamentals of chemical reactions, individuals can appreciate the intricate ways in which matter transforms and evolves.
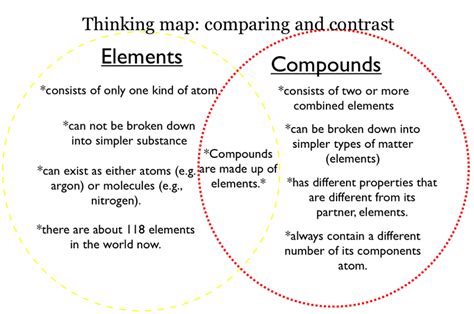
When it comes to chemistry, understanding the properties of elements and compounds is crucial. Elements are the simplest form of matter and cannot be broken down any further, while compounds are made up of two or more elements that are chemically bonded together. These substances have their own unique set of physical and chemical properties that distinguish them from one another.
One way to categorize the properties of elements and compounds is to look at their physical state. Some elements and compounds exist as solids, while others are liquids or gases at room temperature and normal atmospheric pressure. This is a result of differences in the arrangement and bonding of their atoms and molecules.
Additionally, elements and compounds can have varying melting points and boiling points, as well as density and solubility in different solvents. These physical properties are essential to consider when studying the behavior of elements and compounds under different conditions.
Another important aspect of the properties of elements and compounds is their chemical reactivity. Some substances are highly reactive, readily forming new compounds when exposed to other elements or compounds, while others are relatively inert, showing little tendency to undergo chemical changes.
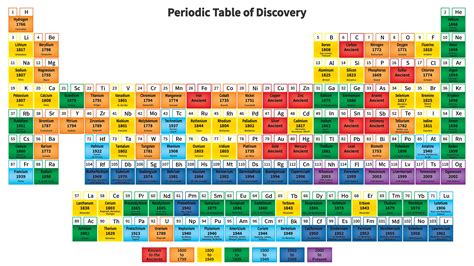
Have you ever looked at the periodic table and felt overwhelmed by all the elements and their properties? Understanding the periodic table is crucial for anyone studying chemistry, and it doesn’t have to be as complicated as it seems. The periodic table is a systematic way of organizing the elements based on their atomic number, electron configuration, and recurring chemical properties.
When you look at the periodic table, you’ll notice that elements are arranged in rows and columns. The rows are called periods, and the columns are called groups. Each element is represented by a chemical symbol and is organized in increasing order of atomic number. This arrangement can help us predict an element’s properties and how it will react with other elements.
One key aspect of the periodic table is the classification of elements into metals, non-metals, and metalloids. Metals are typically found on the left side of the periodic table, while non-metals are on the right. Metalloids are located in between, sharing properties of both metals and non-metals.
Furthermore, understanding the periodic table can help us identify trends in element properties, such as electronegativity, atomic radius, and ionization energy. These trends can provide valuable insights into the behavior of elements and their compounds, making it an invaluable tool for chemists and students alike.
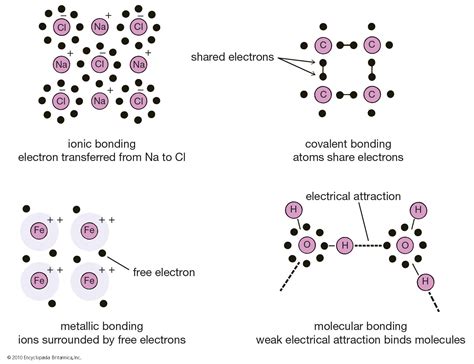
When it comes to the study of chemistry, one of the most crucial concepts to understand is bonding and molecular structures. Bonding refers to the way atoms are held together in molecules, and molecular structures provide insight into the arrangement of atoms within a molecule. These two concepts are fundamental in explaining the behavior and properties of various substances.
There are several types of chemical bonds that can form between atoms, including ionic, covalent, and metallic bonds. Each type of bond has its own unique characteristics and properties, which directly influence the behavior of the substances involved. Understanding these different types of bonds is essential in comprehending the diverse range of compounds and materials that exist in the world around us.
Furthermore, the molecular structure of a compound can have a significant impact on its physical and chemical properties. For instance, the arrangement of atoms in a molecule can determine whether it is a solid, liquid, or gas at a given temperature. Additionally, the shape of a molecule can influence its reactivity and behavior in a chemical reaction. By examining the molecular structures of different compounds, chemists can gain a deeper understanding of their characteristics and behavior.
| Type of Bond | Characteristics |
|---|---|
| Ionic | Transfer of electrons between atoms |
| Covalent | Sharing of electrons between atoms |
| Metallic | Delocalized electrons within a metal lattice |
Chemistry plays a vital role in our everyday lives, whether we realize it or not. From the food we eat to the air we breathe, chemistry is involved in every aspect of our existence. For instance, the process of cooking involves a variety of chemical reactions that transform raw ingredients into delicious meals. Similarly, the medications we take to treat illnesses are formulated using chemical knowledge and principles, ensuring their safety and efficacy.
Moreover, the materials we use in our daily lives, such as plastics, textiles, and metals, are all products of chemical processes. Without chemistry, the modern conveniences we often take for granted would not be possible. In addition, understanding basic chemistry concepts can help us make informed decisions about the products we use and their potential impact on the environment.
Furthermore, chemistry is crucial in fields such as agriculture, manufacturing, and healthcare. In agriculture, pesticides and fertilizers are developed using chemical knowledge to enhance crop yields and protect plants from pests. In manufacturing, chemical processes are instrumental in producing a wide range of products, from electronics to cosmetics. In healthcare, chemistry enables the development of new drugs and medical treatments that improve the quality of life for millions of people.
In conclusion, the importance of chemistry in everyday life cannot be overstated. It is a fundamental science that influences countless aspects of our daily existence, from the products we use to the food we consume. By understanding the role of chemistry, we can appreciate the impact it has on our lives and make informed choices that benefit ourselves and the world around us.

What are the three main subatomic particles?
The three main subatomic particles are protons, neutrons, and electrons.
What is an atom?
An atom is the basic unit of matter, consisting of a nucleus and one or more electrons.
What is the periodic table?
The periodic table is a tabular arrangement of the chemical elements, organized by their atomic number and electron configuration.
What are the chemical elements?
Chemical elements are pure substances that are composed of only one type of atom.
What are molecules?
Molecules are formed when two or more atoms are chemically bonded together.
What is the difference between an element and a compound?
An element is a pure substance made of only one type of atom, while a compound is made of two or more different types of atoms chemically bonded together.
What is the importance of chemistry in everyday life?
Chemistry plays a crucial role in various aspects of everyday life, including medicine, food, and the environment.