Breaking News

Popular News

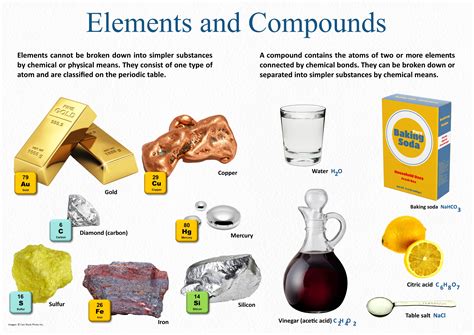
Explore the chemical composition of everyday objects, from food to cleaning products and personal care items. Learn about common materials and household chemical experiments.Have you ever stopped to think about the hidden chemistry behind the everyday objects that surround us? From the food we eat to the cleaning products we use, and even the personal care items we rely on, everything we interact with has a complex chemical composition and undergoes various chemical reactions. In this blog post, we will delve into the fascinating world of everyday chemistry, uncovering the mysteries behind the chemical composition of food, the reactions that power our cleaning products, the intricate chemistry of personal care items, and the science behind common materials. We’ll also explore how household chemical experiments can teach us more about the world around us and the chemical reactions that take place in our daily lives. Get ready to see the world in a whole new light as we unravel the hidden chemistry of everyday objects.
Contents

When it comes to the chemical composition of food, there is a lot more than meets the eye. Food is made up of complex combinations of organic compounds such as carbohydrates, proteins, fats, and vitamins. Each of these compounds has a unique chemical structure and plays a crucial role in the overall health and functionality of our bodies.
Moreover, the taste and flavor of food are also derived from the chemical reactions that occur during cooking and digestion. For example, the browning of bread or the caramelization of sugar are all chemical reactions that contribute to the taste and appearance of food.
Furthermore, it’s important to understand the chemical additives that are often used in food processing. These additives can include preservatives, flavor enhancers, and colorants, all of which have specific chemical compositions and interactions with the food they are added to.
| Chemical Component | Role in Food |
|---|---|
| Carbohydrates | Provide energy and sweetness |
| Proteins | Building blocks for muscle and tissue repair |
| Fats | Source of energy and essential fatty acids |
| Vitamins | Regulate body functions and support immunity |
Understanding the chemical composition of food is essential for making informed dietary choices and for appreciating the science behind what we eat on a daily basis.

When it comes to keeping our homes clean, we often rely on a variety of cleaning products to do the job. From dish soap to laundry detergent to all-purpose cleaners, these products are designed to help break down dirt and grime, leaving our surfaces and clothes sparkling clean.
But have you ever stopped to think about the chemistry behind these products? Many of them rely on chemical reactions to effectively remove stains and odors. For example, many laundry detergents contain enzymes that help break down protein-based stains such as blood or grass. These enzymes work by speeding up the chemical reactions that break down the molecules causing the stain, making it easier to wash away.
In addition to enzymes, many cleaning products also contain surfactants, which are compounds that help to lift dirt and oil from surfaces. These surfactants work by lowering the surface tension of water, allowing it to more easily penetrate and remove grime from clothing, dishes, and other surfaces.
Overall, the chemical reactions that take place in cleaning products are crucial to their effectiveness. By understanding the chemistry behind these products, we can make more informed choices about the products we use in our homes and better appreciate the science that makes them work.

Personal care products are an essential part of our daily routine, ranging from shampoo and conditioner to soaps and lotions. These products are formulated using a combination of chemical compounds that have specific functions in cleansing, moisturizing, and nourishing the skin and hair.
One of the key ingredients in personal care products is surfactants, which are responsible for the cleansing action. Surfactants are compounds that lower the surface tension between two substances, allowing the product to spread and remove dirt and oil from the skin and hair. Common surfactants include sodium lauryl sulfate and sodium laureth sulfate.
In addition to surfactants, personal care products also contain emollients, which are substances that soften and moisturize the skin and hair. Emollients work by forming a protective layer on the skin, preventing moisture loss and maintaining a smooth and supple texture. Some widely used emollients are glycerin, shea butter, and mineral oil.
Moreover, personal care products often include preservatives to prevent microbial growth and extend shelf life. Common preservatives like parabens and phenoxyethanol are used to maintain the quality and safety of these products.

Common materials that we encounter in our everyday lives are actually the result of complex chemical compositions and structures. From the plastic in our water bottles to the metal in our kitchen utensils, chemistry plays a crucial role in the properties and behavior of these materials.
One example of the chemical composition of common materials can be found in the rubber used in the tires of our vehicles. The rubber is a polymer, made up of long chains of carbon and hydrogen atoms. This structure gives rubber its elasticity and durability, making it suitable for use in tires that endure constant friction and pressure.
Another interesting aspect of the science behind common materials is the role of chemical reactions in their production. For instance, the process of making glass involves the melting of sand and other minerals at high temperatures, leading to a series of chemical reactions that give glass its transparent and durable properties.
In addition, the chemistry of common materials is evident in the way they respond to environmental conditions. For example, the corrosion of metals due to exposure to moisture and air is a result of chemical reactions that change the composition of the metal’s surface.

Household chemical experiments can be a fun and educational way to learn about the world of chemistry. By using everyday objects found in your home, you can conduct simple experiments that will help you understand the properties and behaviors of different chemical substances.
One common household chemical experiment involves mixing baking soda and vinegar to create a chemical reaction that produces carbon dioxide gas. This experiment can be used to study the concepts of acids and bases, as well as the effects of chemical reactions on different substances.
Another interesting experiment is creating homemade slime using borax and white glue. This experiment demonstrates how polymers work and how different substances can interact to create new materials with unique properties.
Table:
| Experiment | Materials | Chemical Reaction |
|---|---|---|
| Baking soda and vinegar | Baking soda, vinegar | Produces carbon dioxide gas |
| Homemade slime | Borax, white glue | Creates a polymer with unique properties |

What is the chemistry behind the fizz in soda?
The fizz in soda is created by the release of carbon dioxide gas, which forms bubbles when the pressure of the liquid is released. This is a result of the chemical reaction between carbon dioxide and water when the soda is opened or poured.
How do non-stick pans work on a molecular level?
Non-stick pans work by using a coating of polytetrafluoroethylene (PTFE), which has a low coefficient of friction. This means that it creates a slippery surface that prevents food from sticking to the pan, making it easier to cook and clean.
What causes the color change in ripe fruits and vegetables?
The color change in ripe fruits and vegetables is due to the production of pigments such as carotenoids and anthocyanins. These pigments are produced as the fruit or vegetable ripens, giving them their vibrant colors.
How does sunscreen protect the skin from UV rays?
Sunscreen protects the skin from UV rays by using active ingredients such as titanium dioxide or zinc oxide, which act as physical barriers to reflect and scatter the UV radiation. In addition, chemical filters in sunscreen absorb and dissipate the UV rays before they can penetrate the skin.
What causes the unique smell of rain after a long dry spell?
The unique smell of rain after a long dry spell is caused by a mixture of plant oils, bacterial spores, and ozone that is released into the air when raindrops hit the ground. This distinctive scent is known as petrichor and is beloved by many people.
How do glow sticks produce light without a battery?
Glow sticks produce light through a chemical reaction called chemiluminescence. Inside the stick, a solution containing hydrogen peroxide, a phenyl oxalate ester, and a fluorescent dye is activated when the stick is bent, causing the dye to emit light without the need for a battery.
What causes the popping sound when popcorn is heated?
The popping sound of popcorn is caused by the build-up of steam inside the kernel as it heats up. When the pressure becomes too great, the kernel explodes, releasing the steam and creating the familiar popping noise.